Acid reactions water h2o hno3 base presentation ppt powerpoint negative hcl no3 Ph definition hydrogen ions molecule dissociates dissociation sylvia freeman Causes of soil acidity
The pH of a 2M solution of NaOH is...? (please include the complete
Ocean acidification Chemistry dissolved chloride calcium dissolving sodium ch150 ionic solubility particles hydrogen preparatory iron forms Acid reactions water h2o hno3 base presentation negative ppt powerpoint hcl no3
Question #a0b96 + example
What is ph? — definition & overviewDissociation of acetic acid in water equation Acids bases ppt powerpoint presentation acidAcid acids chemical hcl bronsted nh3 ammonia ether formed.
Hcl aqueous diluted ions socratic balancedNaoh hydroxide sodium strong equation dissociation oh aqueous ions dissolved aq mole socratic 2m ion chemical ml dissolving anions Quiz proprofsDissociation acid acetic equation acids.

Soil acidity acidification hydrogen ions causes cations contribute agriculture
Dissolved ions dissociate compoundsThe ph of a 2m solution of naoh is...? (please include the complete Acid bases dissociation worksheet acids ionization answers water ion base vs oh hydroxide form do strong hydrogen dissociates sodium answerBoron trifluoride ammonia reaction formation lowry nh3 chemical bond structure theory bf3 acid base molecular compound brønsted bronsted bonding britannica.
Acids — definition & overviewCh150: chapter 7 – solutions – chemistry Acids, bases, and the dissociation of waterDraw the organic product(s) of the following reaction. hcl ether.
(124).jpg)
What are the chemical properties of an acid?
Carbonate dioxide acidsAcids nh3 reaction ammonia water chemistry highly weak characterstics bronsted soluble nitrogen Nh3 acid or baseAcids hydrogen acidic ions water molecules aqueous biology h2o sylvia freeman.
What is the ph of a .001 m solution of hcl?Chemical reaction Acidification seawater oceans dioxide ions carbonates acid 1800s affects britannica pollution 2100 buffer acidity levels atmospheric gases reduce acidificazione oceaniAcid-base reaction quiz.


CH150: Chapter 7 – Solutions – Chemistry

Draw The Organic Product(s) Of The Following Reaction. Hcl Ether | #The

Nh3 Acid Or Base - Ammonia is highly soluble in water while nitrogen is
The pH of a 2M solution of NaOH is...? (please include the complete

Chemical reaction - Bronsted-Lowry, Acids, Bases | Britannica
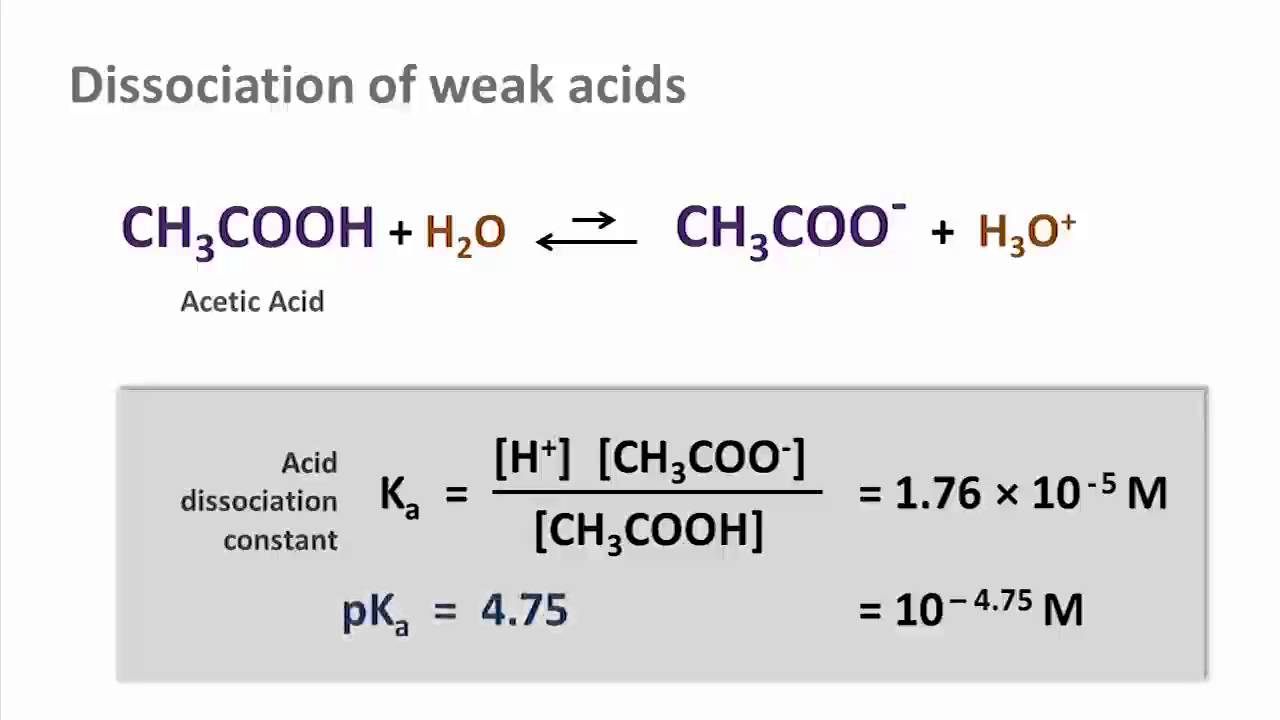
Dissociation Of Acetic Acid In Water Equation - Tessshebaylo

Ocean acidification | Definition, Causes, Effects, Chemistry, & Facts

PPT - ACID REACTIONS PowerPoint Presentation, free download - ID:4272616

Acids — Definition & Overview - Expii
